Group 16 -6
11. Answer the following:
(i) Write the general electronic configuration of VIA or
16th group .
(li) Name two chalcogens.
(iii) Which element of VIA group has maximum catenation property?
(iv) Name the hydride of VIA group elements which is liquid at room temperature.
(v) Which allotropic form of sulphur is more stable at room temperature?
(vi) Which gas is formed in the upper layers of atmosphere by action of UV radiations?
(vii) The common oxidation states of VIA group elements are ...... .
(viii) What is the other name of peroxy disulphuric acid?
(ix) Name theacid used in storage batteries.
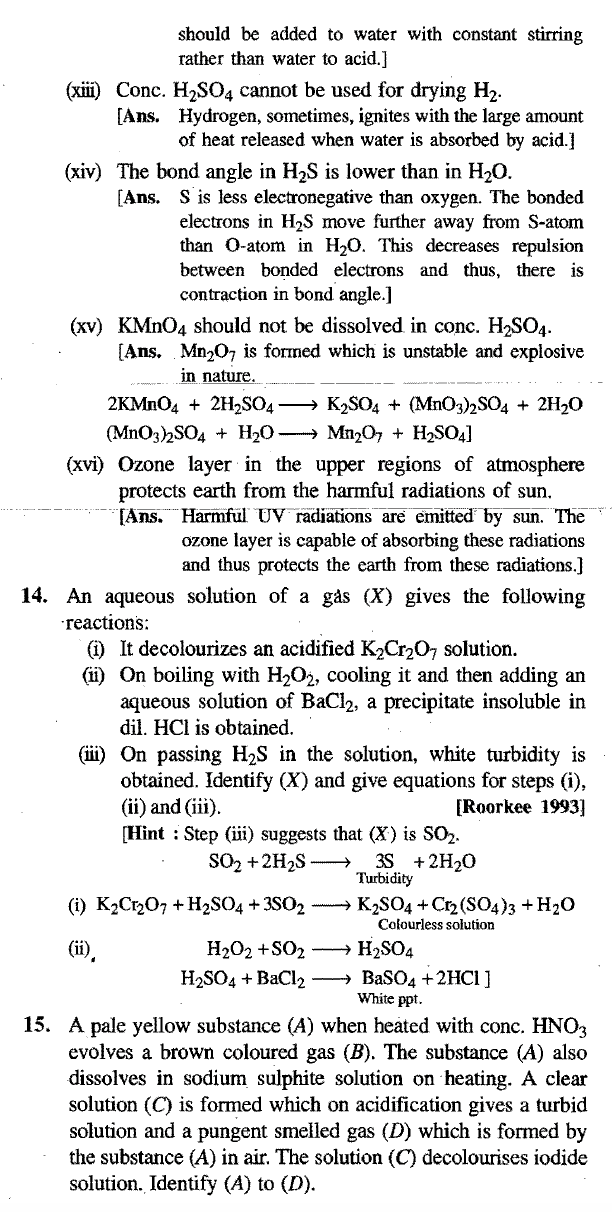
(x) What is the reason for the syrupy nature of conc. H2S04?
(xi) Among the hydrides, H20, H2S, H2Se, which has the highest bond anglr?
(xii) Write the formula of oleum.
(xiii) Which catalyst is used in the manufacture of H2S04 by contact process? [M.L.N.R.1997]
(xiv) What are the most favourable conditions of temperature- andpressure for the conversion-orS021ntoSO~?
(xv) The other name of sulphuric acid is ..... .